Solid-state technology: the new battery frontier for bevs
The engine is frequently the most crucial component in an internal combustion vehicle's performance nowadays. But with BEVs, that argument is irrelevant—and not just because they frequently have a diversity of unusual parts and possibly some storage. The battery is the single most significant component for modern consumers thinking about buying a BEV.
Lithium-ion battery packs power the electric motors that propel all mass-market BEVs today. These batteries are huge and heavy, frequently taking up the entire car's floor; some even engulf the transmission tunnel and a piece of the trunk. In addition to being susceptible to temperature extremes, modern batteries still charge slowly compared to gas fill-ups, deteriorate with time, and occasionally erupt into terrifyingly powerful infernos. Although the architecture of lithium-ion batteries has improved significantly in recent years (resulting in fewer individual cells exploding), fire will always remain a risk due to their fundamental structure. However, brand-new battery technology is currently in research that has the potential to transform the performance of BEVs by producing battery packs that provide more energy while being lighter and less explosive. Solid-state batteries have a lot of potential, but there are still a lot of obstacles to get in the way before they can be commercialized.
.png)
Lithium-ion vs solid-state batteries: how do they work
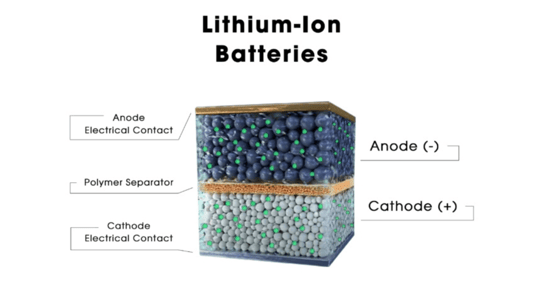
Lithium-ion battery
The majority of batteries use the same fundamental structure, including conventional lithium-ion batteries and solid-state batteries (which also use lithium ion as their core chemistry). The cathode, which acts as the positive terminal during discharging, is located on one side. The anode, the negative terminal, resides on the other side. Depending on the battery type, the internal process varies, but in general, a reaction results in the movement of electrons from one side to the other, forming a circuit that powers your car. The anode and cathode are sandwiched right near to one another, and in the case of cylindrical batteries, they are often even looped around one another, because the lithium-ion batteries in a BEV are intended to be as tiny as possible. An electrical short would result from allowing these two parts to touch, which is really bad news. Consequently, a membrane known as a separator is sandwiched in between to keep things apart. Typically, it is a thin plastic film. However, ions must go from the anode to the cathode, or vice versa, depending on whether the battery is supplying power or being recharged, in order for it to function. How can you make them pass through a plastic divider smoothly? Make careful to use a liquid electrolyte solution.
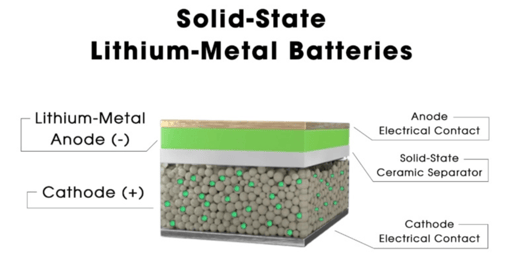
Solid-state battery
The anode and cathode with a separator in between are conceptually the same in a solid-state battery. But in this instance, the separator is the electrolyte, a solid, frequently ceramic substance that permits the direct movement of ions. Although it seems like a small alteration, it has significant repercussions, both good and bad. So, let's go through some solid-state benefits.
Pro
- Rapid Charging
In principle, solid-state batteries ought to be able to charge much more quickly than lithium-ion batteries, depending on how thin the electrolyte can be made to be, without the worries about safety. While solid-state battery pack forecasts vary greatly, several startups in the field believe that a complete charge takes 10 to 15 minutes. With today's average lithium-ion batteries, a full charge can easily take an hour or longer on a fast charger. - High Stability
Generally speaking, a BEV with lithium-ion batteries is completely secure. However, if a battery begins to overheat as a result of damage or poor charging, it could set off a series of events. The electrolyte liquids that are used to fill the batteries may be highly combustible. You experience reactions with that liquid when a battery enters thermal runaway. These chain reactions produce a great deal of heat, which accelerates the reactions even further and results in a fire. Since solid-state batteries don't contain liquid electrolytes, there is less chance of fire even while charging at extremely high rates. - Rapid Construction
The process of making a lithium-ion battery might take a while. The liquid electrolyte is applied during the cell's filling and conditioning phase after it has been built. You can cut your manufacturing line's processing time by up to three weeks by using a solid-state separator instead of those processes. Taking three weeks out of the whole manufacturing process for a car would be significant in today's world of quick manufacturing and just-in-time supply logistics.
Cons
The market for solid-state batteries is being developed by dozens of companies, several of whom have received significant finance from leading OEMs and have optimistic timelines for product introductions in 2025. Though it might be overly hopeful. Let's examine a few of the obstacles.
- Materials scarcities
Lithium is a crucial component in the majority of batteries, even though the interior components differ depending on the construction. Despite the fact that lithium output has tripled merely in the last five years, lithium prices have increased thrice globally in the last year alone. Simply put, there is a global shortage of the material. The issue is that lithium-ion batteries of today could really consume less lithium than solid-state batteries. Do you recall the higher-density anodes we described earlier? Most likely, they'll be made of pure lithium metal. The specific energy of your battery can now be enhanced by up to three times by using lithium metal, but since it is pure lithium, the lithium intensity is also raised. - Recycling questions
Therefore, there are currently no viable methods for recycling solid-state packets. One of the problems with solid-state is that lithium recycling will need to improve. Currently, nickel, cobalt, manganese, aluminium, and copper from the cell components can all be recycled successfully with lithium-ion batteries. The problem, though, is lithium and graphite. They are the bottleneck in closed-loop, bulk battery recycling. The effectiveness of it is still being figured out. Recycling might help resolve supply chain problems while allaying environmental worries, but it might not be implemented soon enough to make a difference. There is a great deal of criticism of materials, but perhaps it hasn't been taken as seriously as it should be. Due to the industrial side of battery recycling's importance for LCA (life cycle assessment), not necessarily an environmental impact given that the recycling processes are quite energy intensive on their own, but rather for easing supply chain stress, there is a messy race to get ahead. It is necessary. Without recycling, we won't be able to produce all of these batteries. - Dendrites
The anode and cathode in solid-state batteries are separated by a solid electrolyte. This calls for more compact, dense batteries as well as an electrode made of pure lithium with a higher density. All is well thus far, right? Researchers have discovered an issue, though, and it is one that also affects the lithium-ion batteries that power today's EVs, particularly when they are frequently charged at high-power, fast-charge stations. The lithium electrode in these batteries has been altering and developing in odd, organic ways as they get older. Dendrites, which are metal branching structures that literally grow into the solid electrolyte, are what lithium is producing.
Eventually, the dendrites lengthen to the point where they can pass through to the electrolyte's opposite side and short out the battery pack. That's awful news once more. The dendrites arise as a result of internal tensions within the battery design, according to a recent MIT study. Those researchers discovered they could stop the dendrite growth by imposing additional physical forces. Although these findings are recent from the lab, it might be years before a fix can be used in mass production. - Cost-competitive when the technology will improve
Cost is arguably the biggest drawback of all. In addition to requiring larger densities of rare metals, solid-state batteries are built entirely differently from today's lithium-ion cells. This indicates that new production facilities, processes, and advantages are still being developed. But eventually, it's possible that these batteries will cost even less. The initial solid-state battery will cost more than (current) lithium-ion batteries and will not be cost-competitive with them. However, the advantages in terms of safety, driving range, and other factors would probably make up for it. The first five to ten years after commercialization, as the technology advances, are when it will start to become cost-competitive. Within the next few years, solid-state batteries are apparently being produced by some automakers, although it is obvious that this will begin on a very small scale. Therefore, 2030 is an optimistic prediction for when the first solid-state batteries will start to be widely available for consumer purchase if we're talking about mass manufacture. Additionally, those initial vehicles will be extremely costly testbed systems. They'll be high-performance, but the point of those cars would be to familiarize themselves with the technology; until they've sort of mastered what the technology is like in the natural EV application, they won't want to manufacture many of those. Finally, a more feasible timeframe for the mass production of solid-state battery electric vehicles is 2032 to 2035. This gives the battery industry about ten years to address the recycling and supply chain challenges, and the focus now must be on reducing battery size and creating batteries that meet consumer needs.
.png?h=628&iar=0&w=1200)