From reprocessing to remanufacturing
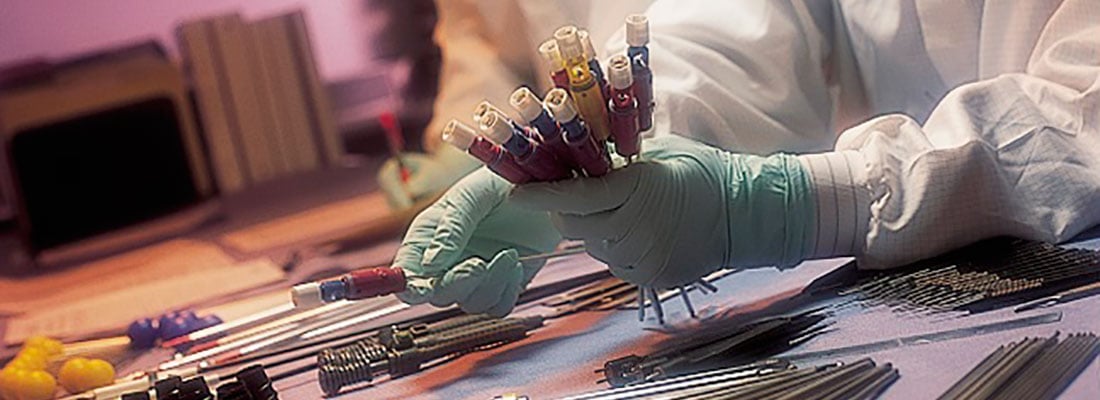
Reprocessing of medical devices is big business. But Daniel J. Vukelich, of the US-based Association of Medical Device Reprocessors, knows he can learn much from remanufacturers in other sectors.
One of the panel discussions at Rematec 2017 in June will take a cross-sectoral approach to reman, looking at issues such as regulatory obstacles, intellectual property, reverse logistics and core management from a variety of points of view. Daniel J. Vukelich, president of the US-based trade body Association of Medical Device Reprocessors (AMDR), will be taking part. AMDR’s mission is to promote the proper reprocessing or remanufacturing (cleaning, repair/refurbishing, testing and sterilization, among other steps) of ‘single use’ devices (SUDs). These come in three main categories: cardiovascular devices, operating room instruments and non-invasive devices (such as blood oxygenation sensors or blood pressure cuffs). Their life can be extended, reducing demand for new devices, eliminating medical waste and repurposing valuable materials such as gold, platinum, steel and plastics. AMDR members perform the majority of the commercial reprocessing in the US and serve over 1,000 European hospitals – including 95% of German university medical centres. The US Food and Drug Administration (FDA) has regulated the industry since 2000, and the sector has quantified its value: the average hospital in the 2016 Practice Greenhealth sustainability benchmark report saved $295,238 annually through using reprocessed devices.
Cost savings
Vukelich insists: “Today many of the US hospitals with whom AMDR members work save more than $1 million annually through programmes that depend upon FDA-approved reprocessed medical devices. Additional cost savings of reprocessed single-use devices include a reduction in medical waste, allowing hospitals to divert millions of pounds from local landfills each year.” Changes are coming to the European Union, he goes on. “In the EU, SUD reprocessing has historically happened in hospitals and been subject to national rules,” Vukelich explains. “With the soon-to-be-adopted European Medical Device Regulation, the reprocessing of SUDs will be held to medical device manufacturer standards. The regulations will likely have the potential impact of stopping hospital reprocessing and encouraging the development of regulated, commercial firms, as the new reprocessing standards are quite stringent.” Reprocessors in the US already have to submit data to the FDA proving that their devices are as clean, as sterile and as functional as their OEM counterparts – and Vukelich says they intend to do the same with notified bodies in Europe going forward.
Proving that remanufacturers in all sectors may have something to learn from one another, the difficulties he describes sound familiar: “Because reprocessing lowers the costs per device by as much as 50%, we’ve found that some OEMs are pushing back on hospitals and inserting contracting language and terms – such as minimum purchase requirements and a blended device approach (new and reprocessed devices bundled together) – that reduces the cost savings to hospitals and weakens the financial and environmental impact of reprocessing.” Despite this, Vukelich is confident that the reman of medical devices is a growth area. “Yes! Our industry is growing worldwide,” he concludes. “We have seen various analyst firms put figures out but, generally, market growth is estimated to be between 10% and 25% year on year.”
Share your remanufacturing stories with us
Do you have an innovation, research results or an other interesting topic you would like to share with the remanufacturing industry? The Rematec website and social media channels are a great platform to showcase your stories!
Please contact our Brand Marketing Manager.
Are you an Rematec exhibitor?
Make sure you add your latest press releases to your Company Profile in the Exhibitor Portal for free exposure.